[Courtesy of Abclon]
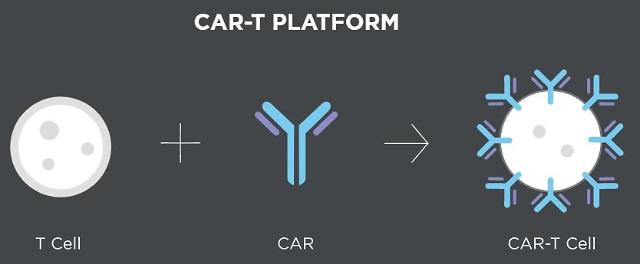
CAR-T cell therapy uses T cells engineered with chimeric antigen receptors (CARs), which are receptor proteins that have been engineered to give immune cells the new ability to target a specific protein. T cells are administered back to the patient after undergoing a mass culture process. Because the therapy activates the patient's immune system, it causes fewer side effects.
By mobilizing their platform technologies, the National Cancer Center and Abclon would develop a CAR-T cell therapy targeting Claudin (CLDN) 18.2., a tight-junction protein. Abclon has used an antibody discovery platform to develop an experimental CAR-T therapy called AT-101 for blood cancers.
"Claudin 18.2 is characterized by overexpression in stomach and pancreatic cancers so it is judged as a strong target to solve the unmet needs of patients with intractable solid cancer," Chung Jun-ho, a professor of biomedical sciences at Seoul National University College of Medicine, said in a statement on April 8.
With the breakthrough success of CAR-T cell therapy for blood cancers, research is actively underway around the world to expand it to intractable solid cancers, but no positive clinical results have been reported due to various difficulties such as the micro-tumor environment of solid cancers.
The National Cancer Center will develop a platform technology that activates the killing ability of T cells only for cancer cells as well as source technology for gene therapy using CAR-T cells that target antigens that exist only in cancer cells. Abclon develops a CAR-T cell therapy for specific antigen Claudin 18.2 and applies it to metastatic solid cancers that do not comply with existing treatments.
"We intend to provide patients with various treatment opportunities by successfully developing a solid cancer CAR-T cell therapy," an unnamed Abclon official was quoted as saying. "This joint research project with the National Cancer Center will be an opportunity for Abclon to become a leading company in global CAR-T cell therapy."
Copyright ⓒ Aju Press All rights reserved.




