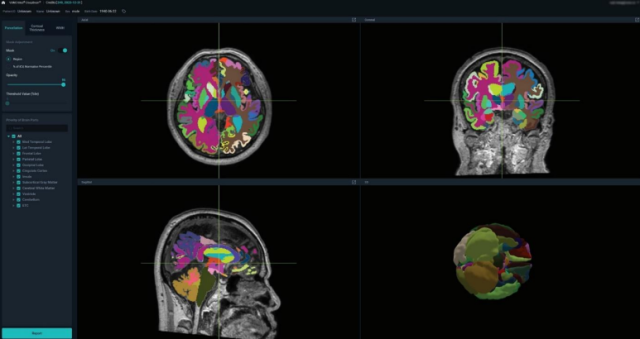
VUNO Med-DeepBrain divides brain magnetic resonance imaging (MRI) images into some 100 sections and provides information on brain atrophy. According to VUNO, the medical device can also be used to diagnose patients experiencing subjective cognitive decline (SCD), an early stage of dementia that worsens cognitive functioning. Memory loss is one of SCD's main symptoms.
VUNO said in a statement that the solution obtained approval from the U.S. FDA. Starting with the approval, the South Korean company aims to make a foray into the American market and partner with global pharmaceutical firms. "As the demand for early diagnosis has increased because of the emergence of next-generation dementia treatments, we will try hard to upgrade our sales capacity so that the product can be rapidly popularized in the U.S. market and help solve dementia problems," VUNO CEO Lee Ye-ha said in a statement on October 10.
South Korea has stepped up the development of AI-based robot doctors to efficiently analyze and diagnose the condition of patients based on medical records. In October 2021, South Korea's state research institute developed "Dr. AI," a robot doctor that can collect digitalized medical records and come up with diagnoses and predictions.
Copyright ⓒ Aju Press All rights reserved.