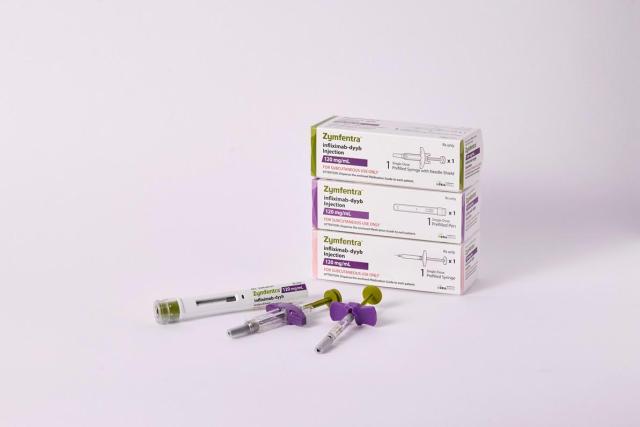
The listing marks a significant milestone for the South Korean biopharmaceutical company as it seeks to accelerate sales of the autoimmune disease treatment in the world's largest pharmaceutical market.
Cigna operates Express Scripts, one of the three largest pharmacy benefit managers in the United States, and Cigna Healthcare, a top-10 U.S. insurer.
Celltrion's U.S. unit had previously secured a contract with Express Scripts to list Zymfentra as a preferred medication. The Evernorth listing builds on that foundation, allowing Cigna-affiliated insurance subscribers to access Zymfentra without the complex administrative procedures typically required for prescription medications.
Since its U.S. launch in 2024, Zymfentra has recorded an average monthly prescription growth rate of 31 percent. Institutional prescriptions, including those from hospitals, surged nearly fivefold in December compared with the same month a year earlier.
"With both Zymfentra and Inflectra now listed on major U.S. insurer formularies, we expect sales growth to accelerate sharply based on our product competitiveness and prescriber preference," a Celltrion official said.
Celltrion plans to leverage its expanding autoimmune disease portfolio, including recently launched biosimilars Steqeyma and Avtozma, to capture greater market share in the United States through intensified marketing efforts.
Copyright ⓒ Aju Press All rights reserved.