The pharma plans to invest about 5.4 trillion won ($3.67 billion) in production facilities across South Korea and the United States to ramp up biosimilar manufacturing capacity and strengthen its novel drug pipeline.
Part of the funding will be channeled into the development of "a quadruple-action obesity drug that is one step more advanced than existing GLP-1 and dual- and triple-agonists," said Celltrion Chairman Seo Jung-jin, referring to blockbuster therapies such as Novo Nordisk's Ozempic and Wegovy.
"I don't believe the Wegovy era will last forever," Seo said during an online briefing Wednesday.
Seo said the company expects to complete derivation of three candidate compounds by year-end. The experimental drug, designated CT-G32, is designed to address limitations of earlier treatments, including variations in individual response and muscle-loss side effects.
Obesity treatments have surged in demand after public figures — including Tesla's Elon Musk — publicly highlighted their effectiveness. The drugs have also shown utility in managing coronary heart disease, hypertension and kidney disease, far beyond their original purpose as diabetes medications.
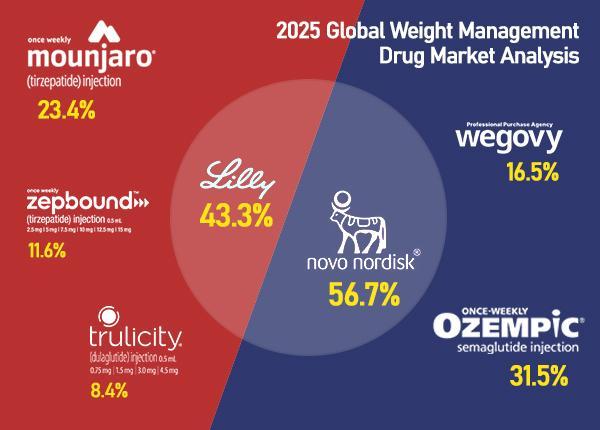
"We believe we are now at an inflection point for the broadening of obesity-drug use, which will extend beyond the U.S. to larger numbers of patients globally," said Morgan Stanley equity analyst Terence Flynn.
To challenge front-runners Novo Nordisk and U.S.-based Eli Lilly, Celltrion is developing a quadruple-action mechanism it says can achieve about 25 percent weight reduction — higher than Ozempic's 5 to 15 percent and Eli Lilly's Mounjaro at 15 to 22.5 percent. It expects the drug to reduce non-response rates to below 5 percent.
The treatment's oral formulation is expected to offer a convenient alternative for patients reluctant to self-inject or seeking a simpler regimen to maintain weight loss. Celltrion expects to complete animal testing by year-end and begin preclinical regulatory studies next year.
Beyond obesity treatments, the company plans to spend up to 700 billion won expanding its U.S. manufacturing base as it navigates uncertainties over potential biosimilar tariffs and doubles down on American production capabilities.
Celltrion is acquiring a 66,000-liter biopharmaceutical production facility owned by Eli Lilly in Branchburg, New Jersey. Including acquisition costs, about 1.4 trillion won will go toward securing U.S. manufacturing capacity.
Domestically, Celltrion will invest about 4 trillion won in new facilities, including drug-substance plants in Songdo, a drug-product facility in Yesan in South Chungcheong Province, and a pre-filled syringe plant in Ochang, North Chungcheong Province.
"As the new drug pipeline expands, R&D spending will reach about 800 billion won next year and 1 trillion won by 2027, but we can secure sufficient cash flow through sales expansion," Seo said.
Analysts expect the company's fourth-quarter performance to maintain momentum.
"New products including Stoboclo and Osenvelt for osteoporosis are expanding sales, while Omlyclo for urticaria and Avtozma for autoimmune conditions are launching with initial shipment volumes — which will drive sales growth in the new-product segment," said Lee Dal-mi, an analyst at Sangsangin Securities.
Shares of Celltrion finished Thursday 0.9 percent higher at 186,800 won.
Copyright ⓒ Aju Press All rights reserved.